Background: Despite several attempts of randomized phase III trial to overcome R-CHOP in overall survival (OS), R-CHOP has been continued to be a standard of care in previously untreated DLBCL. We conducted a randomized phase II/III study (JCOG0601, jRCTs031180139) that investigated the efficacy of dose-dense weekly rituximab combined with standard CHOP regimen (RW-CHOP) during the early treatment period for previously untreated DLBCL and published the results (Ohmachi K, et al. Blood Adv. 2021). Here in, we report the long-term efficacy and safety of the JCOG 0601 trial after 8 years follow-up from the end of accrual.
Methods: Patients aged 20-79 years with previously untreated CD20-positive DLBCL (stage I-IV, performance status 0-2) were randomized to standard R-CHOP (CHOP-21 with eight doses of rituximab, once every 3 weeks) or RW-CHOP (CHOP-21 with eight doses of weekly rituximab). The primary endpoint of phase III part was progression-free survival (PFS). Required sample size was 422 patients, an accrual period of 7 years, and a follow-up period of 3 years with the primary analysis. An additional follow-up period was added to assess long-term outcomes, for a total of 8 years of follow-up.
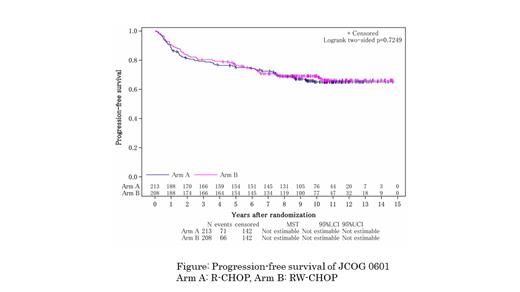
Results: Between December 2007 and December 2014, 422 patients were enrolled, but primary analysis was performed on 421 patients after one patient withdrew consent: 213 in the R-CHOP arm and 208 in the RW-CHOP arm. The baseline characteristics were as follows (R-CHOP arm vs. RW-CHOP arm): median age, 61 vs. 62 years; male sex, 54.5% vs. 55.8%; Ann Arbor stage I/II/III/IV, 14.6/32.9/26.8/25.8% vs. 16.3/42.8/20.2/20.7%; and International Prognostic Index score ≤2, 77.0% vs. 87.5%. At a primary analysis, there was no significant difference in PFS between the arms. At the time of final analysis with a median follow-up of 9.6 years (range: 0.3-14.9) among all patients, meaningful differences were not found in PFS and OS as well as the primary analysist (hazard ratio [HR] in PFS of RW-CHOP against R-CHOP, 0.94; 95% confidence interval [CI], 0.67 to 1.32, one-sided log-rank, P = 0.36 and HR in OS, 0.94; 95% CI, 0.63 to 1.41). Median PFS and OS were not estimable in both arms. Estimated 10 years PFS and OS of all patients was 66.9% (95% CI, 62.1 to 71.3) and 78.0% (95% CI, 73.6 to 81.7). After the first relapse or refractoriness, 126 patients received post-protocol salvage therapy: 66 in the R-CHOP arm and 60 in the RW-CHOP arm. In each arm, 3 patients received high-dose chemotherapy followed by autologous stem cell transplantation. One patient in the R-CHOP arm proceeded to allogeneic stem cell transplantation from a sibling donor. Ann Arbor stage I to II disease tended to have more favor prognosis than Ann Arbor stage III to IV disease, and no sustained relapse was observed in either stage. Of the 401 patients who underwent central pathological review, 125 were diagnosed with germinal center B-cell-like (GCB) type and 216 with non-GCB type by immunohistochemistry analysis. There was no remarkable difference in PFS between the arms for GCB type and non-GCB type. Death occurred in 95 patients: 56 from DLBCL, 2 from treatment-related cardiac toxicity, 9 from treatment-related mortality caused by salvage treatment, 14 from secondary malignancies, and 11 from other diseases or reasons. There were 34 secondary malignancies in 31 patients (14.6%) in the R-CHOP arm, and 39 cases in 35 patients (16.8%) in the RW-CHOP arm. The most common secondary malignancies were lung cancer (2.6%), colon cancer (1.9%), prostate cancer (1.7%) and gastric cancer (1.9%). Three cases of acute myeloid leukemia (0.7%) and three of myelodysplastic syndromes (0.7%) were observed. The median age of patients who developed secondary malignancy was 72 years (range: 49-84) at the onset. The median time from study enrollment to the onset of secondary malignancy was 4.5 (range: 0.1-13.1) years and the incidence was time dependent. There were no unexpected adverse events, including late opportunistic infections.
Conclusion: This final-follow up data demonstrated again no superiority of RW-CHOP in PFS and OS. Standard R-CHOP remains a standard of care for untreated DLBCL.
Disclosures
Ohmachi:Chugai pharma: Honoraria; Novartis Pharma: Honoraria; Meiji Seika Pharma: Honoraria; Janssen Pharma: Honoraria; Kyowa Kirin: Honoraria; Yakuzemi total learning: Honoraria; Genmab: Honoraria; Symbio pharma: Honoraria. Kinoshita:Japanese Red Cross, Director of Aichi Blood Center: Current Employment. Maruyama:Eizai: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; Taiho: Research Funding; Amgen Astellas Biopharma: Research Funding; Kyowa Kirin: Honoraria, Research Funding; Sanofi: Honoraria, Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Honoraria, Research Funding; Novartis: Research Funding; Janssen: Honoraria, Research Funding; Otsuka: Research Funding; Astellas: Research Funding; Abbvie: Honoraria, Research Funding; MSD: Honoraria, Research Funding; Ono Pharmaceuticals: Honoraria, Research Funding; Nippon Shinyaku: Honoraria; Mundipharma: Honoraria, Research Funding; Zenyaku: Honoraria; SymBio Pharmaceuticals: Honoraria; AstraZeneca: Honoraria; Chugai Pharma: Honoraria, Research Funding; Takeda: Honoraria, Research Funding. Yamauchi:Incyte: Research Funding; Ono: Research Funding; Takeda: Research Funding; Genmab: Research Funding. Fukuhara:BMS: Honoraria; Eisai: Honoraria; Janssen: Honoraria; Nippon Shinyaku: Honoraria; AstraZeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Research Funding; Loxo Oncology: Research Funding; Genmab: Honoraria, Research Funding; Kyowa Kirin: Honoraria, Research Funding; Chordia Therapeutics: Research Funding; Chugai Pharma: Honoraria, Research Funding; Bayer: Research Funding; Abbvie: Membership on an entity's Board of Directors or advisory committees, Research Funding; Meiji Seika: Honoraria; Nihon kayaku: Honoraria; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Ono Pharmaceuticals: Honoraria; SymBio Pharmaceuticals: Honoraria; Eli Lilly: Membership on an entity's Board of Directors or advisory committees; HUYA: Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Research Funding. Uchida:AbbVie: Honoraria; AstraZeneca: Honoraria; Nippon Shinyaku: Honoraria; Novartis Pharmaceuticals: Honoraria; Otsuka Pharmaceutical: Honoraria; NovartJanssen Pharmaceutical: Honoraria; Nippon Kayaku: Honoraria; Kyowa Kirin: Honoraria; Bristol Myers Squibb: Honoraria; Meiji Seika Pharma: Honoraria; Takeda Pharmaceutical: Honoraria. Yamamoto:AbbVie Inc.: Honoraria; Chugai Pharmaceutical Co., Ltd.: Honoraria; Eisai Co., Ltd.: Honoraria; HUYA/IQIVA: Honoraria; Janssen Pharmaceutical K.K.: Honoraria; Meiji Seika Pharma Co., Ltd.: Honoraria; Molecular Imaging CRO Network/DAIICHI SANKYO COMPANY, LIMITED: Honoraria; Takeda Pharmaceutical: Honoraria; Genmab/IQIVA: Research Funding; Yakult: Research Funding. Miyazaki:Eisai: Honoraria, Research Funding; Takeda: Research Funding; Nippon Shinyaku: Honoraria, Research Funding; Nippon Shinyaku: Research Funding; Otsuka: Research Funding; Chugai Pharma: Honoraria, Research Funding; Asahi Kasei: Honoraria, Research Funding; Sumitomo Dainippon Pharma Oncology: Research Funding; Zenyaku Kogyo: Research Funding; Chugai Pharma, SymBio Pharmaceuticals, Janssen, Eisai, Nippon Shinyaku, AstraZeneca, Bristol-Myers Squibb Japan, Meiji Seika, Abbvie, Novartis, Incyte, Asahi Kasei, Ono Pharmaceuticals: Honoraria; SymBio Pharmaceuticals: Honoraria; Janssen: Honoraria; AstraZeneca: Honoraria; Bristol-Myers Squibb Japan: Honoraria; Meiji Seika: Honoraria; Abbvie: Honoraria; Novartis: Honoraria; Incyte: Honoraria; Ono Pharmaceuticals: Honoraria. Iida:Shionogi: Research Funding; Otsuka: Consultancy; Regeneron: Consultancy; Alexion: Research Funding; GlaxoSmithKlein: Consultancy, Research Funding; Amgen: Research Funding; Otsuka: Research Funding; Novartis: Consultancy, Research Funding; Chugai: Research Funding; Abbvie: Consultancy, Research Funding; Ono: Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Daiichi Sankyo: Research Funding; Sanofi: Consultancy, Honoraria, Research Funding; Bristol-Myers Scuibb: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Research Funding. Yoshida:Janssen, Eisai, Dainipponsumitomo, BMS, MeijiSeika pharma, NipponKayaku and Astrazeneca: Honoraria; Kyowa Kirin, Chugai: Honoraria, Research Funding. Masaki:Eisai: Honoraria, Research Funding; Takeda: Honoraria, Research Funding; Meiji Seika: Honoraria; Ono: Honoraria; Nippon Kayaku: Honoraria; Novartis: Honoraria; Astellas: Research Funding; Bristol Myers: Honoraria; NIppon Sinyaku: Honoraria; Teijin: Research Funding; Taiho: Research Funding; Japan Blood Product Organization: Research Funding; Astra Zeneca: Honoraria; Sumitomo: Honoraria; Mundi: Honoraria; Chugai: Honoraria, Research Funding; Kyowa Kirin: Honoraria, Research Funding; Asahi Kasei: Honoraria, Research Funding; Abbvi: Honoraria, Research Funding; Otsuka: Research Funding; Daiichi Sankyo: Honoraria, Research Funding; Taisyo Toyama: Research Funding. Murayama:AbbVie GK: Honoraria; Bristol Myers Squibb K. K.: Honoraria; Janssen Pharmaceutical K.K.: Honoraria; Eisai Co., Ltd.: Honoraria; Mundipharma K.K.: Honoraria; Nippon Shinyaku Co., Ltd: Honoraria; Ono Pharmaceutical CO. Ltd..: Honoraria; Kyowa Kirin Co.,Ltd.: Honoraria; SymBio Pharmaceuticals Limited: Honoraria; Takeda Pharmaceutical Company Limited: Honoraria; Novartis Pharma K.K.: Honoraria; Otsuka Pharmaceutical Co., Ltd.: Honoraria; Chugai Pharmaceutical Co.Ltd: Honoraria. Suehiro:Abbvie: Honoraria, Research Funding; Nippon Kayaku: Honoraria, Research Funding; Teijin: Research Funding; Amgen: Research Funding; Otsuka: Research Funding; Kyowa Kirin: Research Funding; Genmab: Honoraria, Research Funding; Incyte: Research Funding; BMS: Honoraria; Nippon Shinyaku: Honoraria; Pfizer: Honoraria; Meiji Pharma: Honoraria; Janssen: Honoraria; Sanofi: Honoraria; Chugai: Honoraria, Research Funding. Nosaka:Chugai Pharmaceutical K.K.: Honoraria, Research Funding; Dai-ichi Sanyo: Honoraria; Meiji Seika: Honoraria; Bristol Myers Squibb: Honoraria; Kyowa Kirin Co.,Ltd.: Honoraria, Research Funding; Abbvie: Honoraria; Eisai: Honoraria. Dobashi:Mundipharma K.K.: Research Funding; Eisai Co., Ltd.: Speakers Bureau; Novartis Pharma K.K.: Speakers Bureau; Otsuka Pharmaceutical Co., Ltd.: Consultancy, Research Funding, Speakers Bureau; AstraZeneca K.K.: Speakers Bureau; Astellas Pharma Inc.: Speakers Bureau; Nippon Shinyaku Co., Ltd.: Speakers Bureau; Janssen Pharmaceutical K.K.: Speakers Bureau; Amgen K.K.: Speakers Bureau; Kissei Pharmaceutical Co., Ltd.: Research Funding; Kyowa Hakko Kirin Co., Ltd.: Research Funding; Daiichi Sankyo Co., Ltd.: Research Funding; Taiho Phamaceutical Co., Ltd.: Research Funding; AbbVie GK.: Research Funding, Speakers Bureau; Chugai Pharmaceutical Co., Ltd.: Research Funding, Speakers Bureau. Kuroda:Bristol Myeres Squibb, Kyowa Kirin, Chugai, Japan Blood Product Organization, Daiichi Sankyo, Mochida, Ono, Sanofi, Eisai, Taiho, Sumitomo, Asahikasei, Otsuka, Takeda, Shionogi Janssen, Novartis, Abbvie, Pfizer, Nippion Shinyaku, Astellas: Consultancy, Honoraria, Research Funding. Munakata:Janssen, Takeda, Celgene, ONO PHARMACEUTICAL, Eisai, CHUGAI, Novartis Pharma, Bristol-Myers Squibb, AstraZeneca, SymBio Pharmaceuticals, Genmab, NIPPON SHINYAKU, Nippon Kayaku, Gilead Sciences, Otsuka Pharmaceutical, Kyowa Kirin: Honoraria, Research Funding. Ando:Nippon Shinyaku: Honoraria, Research Funding; Takeda Pharmaceutical: Research Funding; Chugai Pharmaceutical: Research Funding; Eisai: Honoraria, Research Funding; Kyowa Kirin: Research Funding; Otsuka Pharmaceutical: Research Funding; Novartis: Research Funding; BMS: Honoraria, Research Funding; Asthelas: Research Funding; Janssen: Honoraria; Meiji seika pharma: Honoraria; Nippon kayaku: Honoraria. Ishizawa:AbbVie, Symbaio, ZENYAKU, IQVIA, Novartis: Honoraria, Research Funding. Ogura:Meiji Seika Pharma: Consultancy; SymBio: Consultancy; Yakult: Consultancy. Yoshino:Kyowa Kirin: Honoraria; Chugai Pharmaceutical Co., Ltd.: Honoraria, Research Funding. Hotta:SymBio Pharmateuticals Limited: Consultancy. Tsukasaki:Chugai Pharma: Honoraria; Eisai: Honoraria; Ono Pharma: Consultancy; Meiji Seika Pharma: Consultancy, Honoraria, Research Funding; Daiich-Sankyo: Consultancy, Research Funding; HUYABIO: Consultancy, Research Funding; Bristol Myers Squibb: Research Funding; Byer: Research Funding; Regeneron Pharmaceuticals Inc.: Research Funding; Solasia Pharma: Consultancy; Yakuruto: Consultancy; Kyowa kirin: Research Funding. Tobinai:HUYA Bioscience International: Consultancy, Honoraria; Celgene: Honoraria; Daiichi Sankyo: Consultancy, Honoraria; Mundipharma: Consultancy, Honoraria; Solasia Pharma: Honoraria; Zenyaku Kogyo: Consultancy, Honoraria. Nagai:Chugai: Honoraria, Research Funding; Mitsubishi Tanabe: Research Funding; Astra Zeneka: Honoraria, Research Funding; HUYA: Research Funding; Janssen: Honoraria, Research Funding; Eli Lilly: Honoraria, Research Funding; Genmab: Honoraria, Research Funding; MSD: Honoraria, Research Funding; Kyowa Kirin: Honoraria, Research Funding; Takeda: Honoraria, Research Funding; Daiichi Sankyo: Research Funding; BMS: Honoraria; Zenyaku Kogyo: Research Funding; Solasia: Research Funding; Ono: Honoraria, Research Funding; Eisai: Honoraria; Novartis: Honoraria; Sumitomo Pharma: Honoraria; Meiji Seika Pharma: Honoraria; Mundi pharma: Honoraria; GSK: Honoraria; Celgene: Research Funding; Abbvie: Honoraria, Research Funding; Beigene: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal